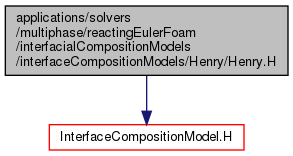
Go to the source code of this file.
|
| class | Henry< Thermo, OtherThermo > |
| | Henry's law for gas solubility in liquid. The concentration of a dissolved species in the liquid is proportional to its partial pressure in the gas. A dimensionless solubility, 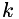 , is given for each species. This is the ratio of the concentration of the species in the liquid to the corresponding concentration in the gas; i.e., , is given for each species. This is the ratio of the concentration of the species in the liquid to the corresponding concentration in the gas; i.e., 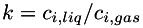 . Mixing in the gas is assumed to be ideal. More... . Mixing in the gas is assumed to be ideal. More...
|
| |
Original source file Henry.H
Definition in file Henry.H.
 , is given for each species. This is the ratio of the concentration of the species in the liquid to the corresponding concentration in the gas; i.e.,
, is given for each species. This is the ratio of the concentration of the species in the liquid to the corresponding concentration in the gas; i.e., 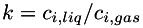 . Mixing in the gas is assumed to be ideal. More...
. Mixing in the gas is assumed to be ideal. More...