|
| class | Henry |
| | Henry's law for gas solubility in liquid. The concentration of a dissolved species in the liquid is proportional to its partial pressure in the gas. A dimensionless solubility, 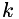 , is given for each species. This is the ratio of the concentration of the species in the liquid to the corresponding concentration in the gas; i.e., , is given for each species. This is the ratio of the concentration of the species in the liquid to the corresponding concentration in the gas; i.e., 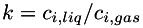 . Mixing in the gas is assumed to be ideal. More... . Mixing in the gas is assumed to be ideal. More...
|
| |
| class | NonRandomTwoLiquid |
| | Non ideal law for the mixing of two species. A separate composition model is given for each species. The composition of a species is equal to the value given by the model, scaled by the species fraction in the bulk of the other phase, and multiplied by the activity coefficient for that species. The gas behaviour is assumed ideal; i.e. the fugacity coefficient is taken as equal to 1. More...
|
| |
| class | Raoult |
| | Raoult's law of ideal mixing. A separate composition model is given for each species. The composition of a species is equal to the value given by the model scaled by the species fraction in the bulk of the other phase. More...
|
| |
| class | Saturated |
| | Model which uses a saturation pressure model for a single species to calculate the interface composition. More...
|
| |
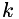 , is given for each species. This is the ratio of the concentration of the species in the liquid to the corresponding concentration in the gas; i.e.,
, is given for each species. This is the ratio of the concentration of the species in the liquid to the corresponding concentration in the gas; i.e., 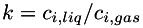 . Mixing in the gas is assumed to be ideal. More...
. Mixing in the gas is assumed to be ideal. More...