Henry's law for gas solubiliy in liquid. The concentration of the dissolved species in the liquid is proportional to its partial pressure in the gas. The dimensionless constant of proportionality between concentrations on each side of the interface is 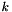 , and is given for each species. Mixing in the gas is assumed to be ideal.
More...
, and is given for each species. Mixing in the gas is assumed to be ideal.
More...
Henry's law for gas solubiliy in liquid. The concentration of the dissolved species in the liquid is proportional to its partial pressure in the gas. The dimensionless constant of proportionality between concentrations on each side of the interface is 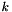 , and is given for each species. Mixing in the gas is assumed to be ideal.
, and is given for each species. Mixing in the gas is assumed to be ideal.
 1.8.13
1.8.13