Go to the source code of this file.
Classes | |
| class | Henry |
Henry's law for gas solubility in liquid. The concentration of a dissolved species in the liquid is proportional to its partial pressure in the gas. A dimensionless solubility, 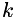 , is given for each species. This is the ratio of the concentration of the species in the liquid to the corresponding concentration in the gas; i.e., , is given for each species. This is the ratio of the concentration of the species in the liquid to the corresponding concentration in the gas; i.e., 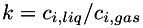 . Mixing in the gas is assumed to be ideal. More... . Mixing in the gas is assumed to be ideal. More... | |
Namespaces | |
| Foam | |
| Namespace for OpenFOAM. | |
| Foam::interfaceCompositionModels | |